Hydrogen as a sustainable solution to green steel making

On average globally, for every tonne of steel produced 1.9 tonnes of CO2 are being emitted, accounting for about 5% of the global CO2 emissions. Apart from these 2.8 million tonnes of CO2 is being emitted through the energy used in the iron and steel industry. This has become a serious concern globally and hence huge investments are being made to use the eco-friendly method of steel production. EU is the world’s second-largest producer of steel after China (11% of the global steel output) producing over 177 million tonnes of steel a year. Even though steel can be efficiently recycled without any carbon emissions, the growing demand for high-grade steel and less availability of scrap iron makes it not a sustainable solution for carbon-neutral steel production. Carbon plays an important role in the steel industry making it a hard-to-decarbonize sector. Transitioning to a green economy also requires a change in the way we manufacture iron and steel. In this regard, shifting to hydrogen-based steel production technologies will help in making the iron and steel industry carbon-neutral. Hence, this article gives you an understanding of how hydrogen could help decarbonize the iron and steel industry.
Primary steel making
Traditionally steel is made from molten pig iron or crude iron produced by heating iron ore in a blast furnace. A blast furnace is a furnace used for the smelting of iron ore into molten pig iron. First coal is converted into coke also known as pyrolyzed coal in a plant by heating it in an oven at a high temperature of about 1100 °C in the absence of air, making the steel industry the largest user of coal as coal is not only directly used in the process but also acts as a source of heat needed throughout the entire process. This coke along with sintered iron ore or pellets and limestone is fed into a blast furnace at high temperatures of about 1650 °C. At this high temperature, the carbon from coke and carbon monoxide from coal combustion undergoes direct and indirect reduction reaction with the ore respectively as shown below.
C + O2 —> CO2 (exothermic)
CO2 + C ↔ 2CO (endothermic)
3Fe2O3 + CO —> 2Fe3O4+ CO2 (hematite —> magnetite)
Fe3O4 + CO —> 3FeO + CO2 (magnetite —> wüstite)
FeO + CO —> Fe + CO2 (wüstite —> iron)
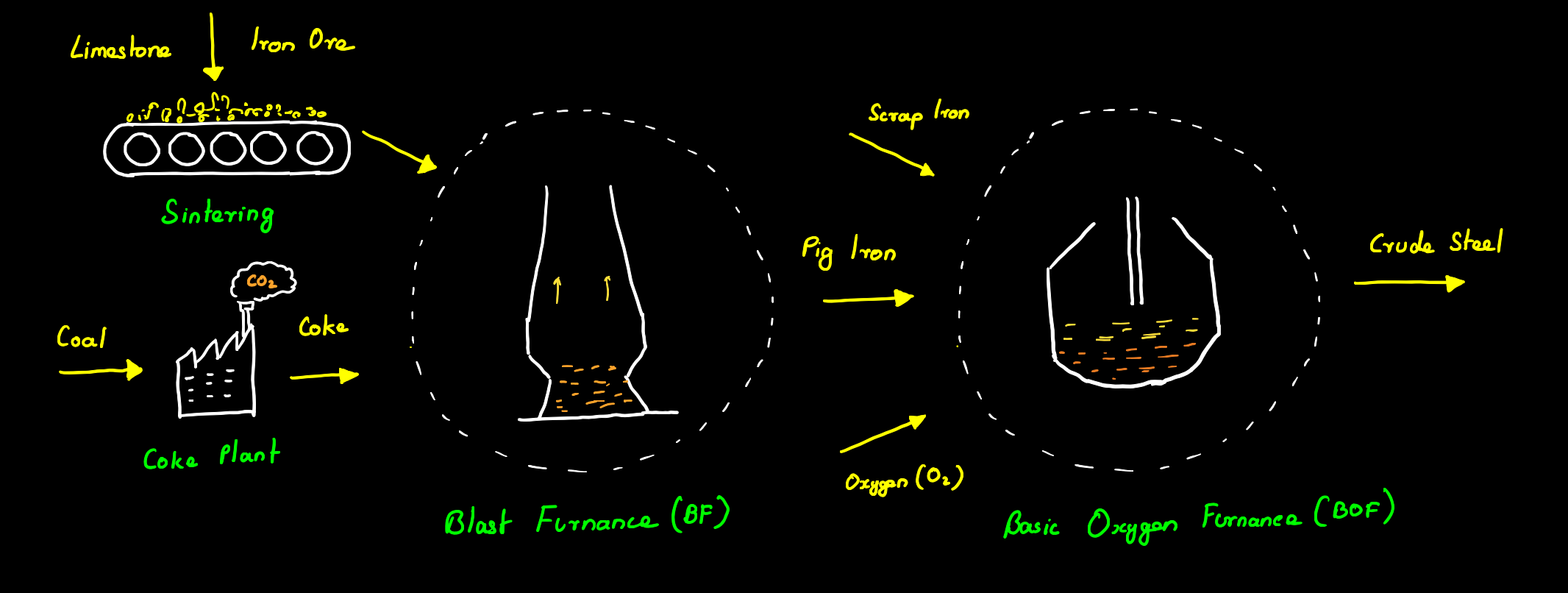
A large amount of CO2 is released in the entire process coming from both the formation of pig iron and from the burning of coal for heat generation. This pig iron formed in the blast furnace is brittle due to high carbon (3.8 – 4.7 %) and impurities like silica. This pig iron is then passed into a Basic Oxygen Furnace (BOF) where oxygen is blown through the molten pig iron thereby reducing the carbon content and forming low-carbon steel. About 67% of the global crude steel is produced through the BOF method.
Secondary steel making
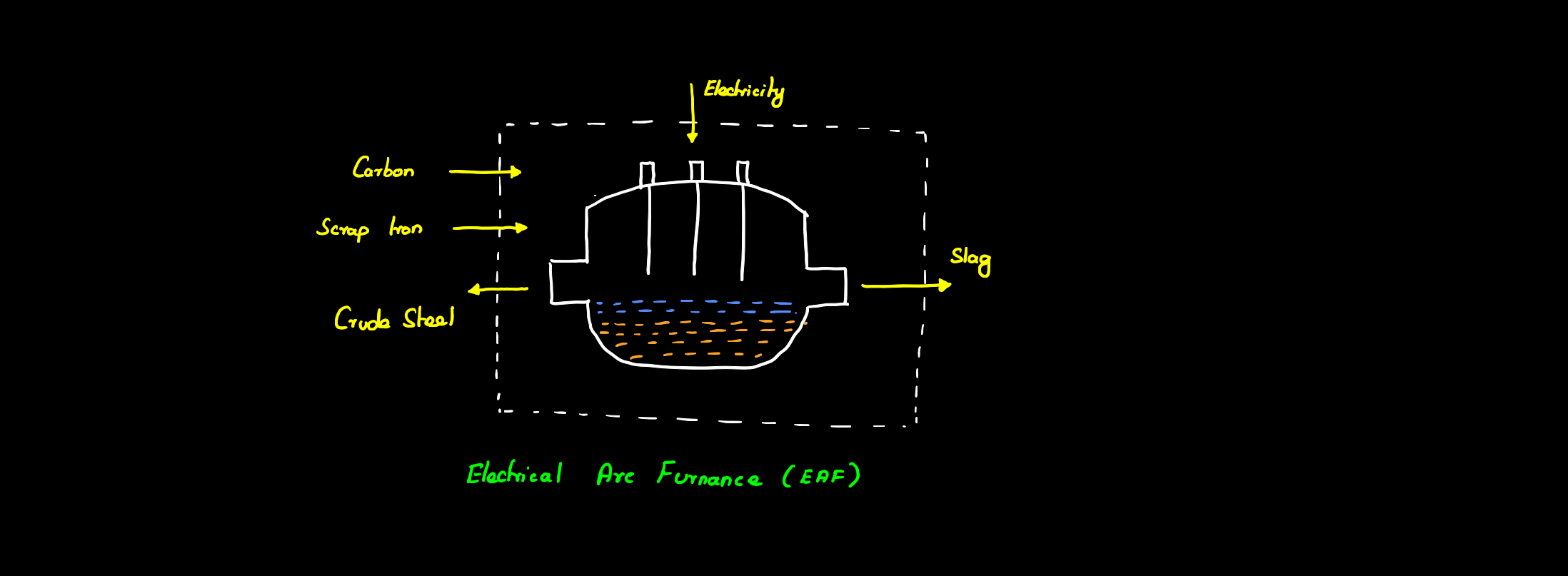
Steel is also traditionally recycled using an electric arc furnace (EAF) where scrap iron is heated using electric arcs in an oven to form steel. The greenhouse gas emissions from this process are dependent on the energy source used for producing the required electricity. Apart from this, the EAF method is also limited due to the less availability of scrap iron and increasing demand for high-grade steel as EAF cannot be used to produce the required quality of steel. 50% of the steel in Europe is produced from scrap iron using this method. To cope with the demand and also reduce the carbon emissions from conventional steel making several technologies are being developed to use green hydrogen for steel production some of which are discussed below.
Hydrogen based steel making
Hydrogen-based steel making in principle uses hydrogen instead of carbon as the reducing agent thereby eliminating harmful carbon emissions. The hydrogen gas directly reduces the iron ore to iron and then carbon is later added to produce steel. There are several technologies being developed for the efficient reduction of iron ore using hydrogen, some are more promising than others whereas some are more efficient than others.
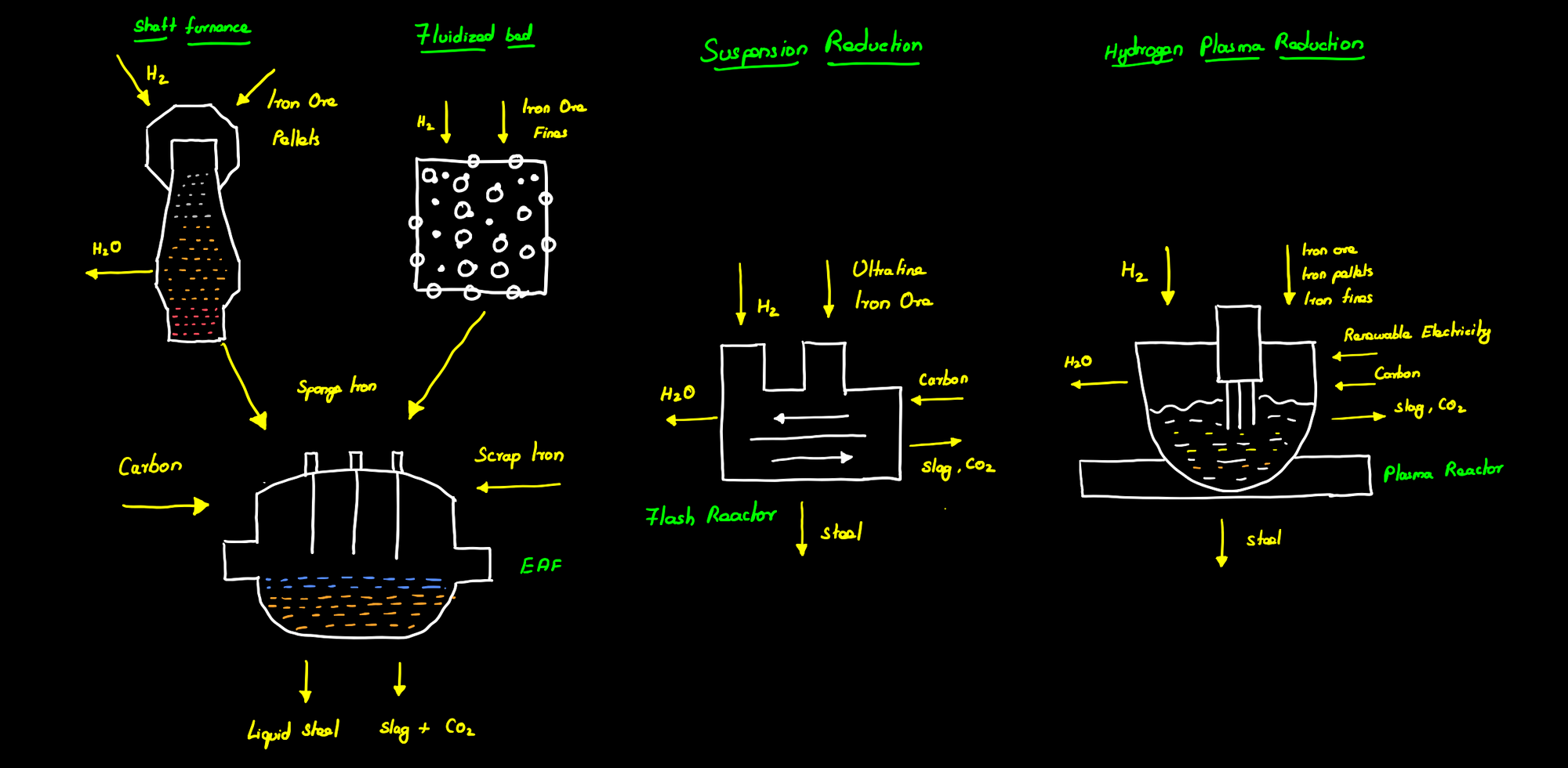
Direct reduced iron – Shaft furnace
Direct reduced iron is the process of chemically reducing the iron ore directly without melting it. The iron ore pellets are passed into a shaft furnace where they are heated to a high temperature of 800 to 1000 °C. Hydrogen gas is then used to remove the oxygen content from the ores resulting in cracks on the iron ore surface.
3Fe2O3 + H2 —> 2Fe3O4 + H2O (hematite —> magnetite)
Fe3O4 + H2 —> 3FeO + H2O (magnetite —> wüstite)
FeO + H2 —> Fe + H2O (wüstite —> iron)
The reduced iron ore resembles a porous sponge and hence is called sponge iron. The direct reduced iron or Sponge iron is then fed into an electric arc furnace (EAF) along with scrap iron and turned into steel by further processing and the addition of carbon.
Direct reduced iron – Fluidized Bed reactor
The fluidized bed-based DRI is similar in operation to the Shaft furnace-based DRI except that the reduction of the iron ore takes place in a Fluidized bed reactor. A fluidized bed reactor is a chamber that continuously mixes the iron ore powder with hydrogen to produce sponge iron. This sponge iron is then fed to an EAF to produce steel through the addition of carbon. At temperatures below 843 k (570 °C), the direct reduction proceeds from hematite to magnetite and then directly to metallic iron as wüstite is not stable below 843 k whereas at temperatures above 843 K (570 °C) the reduction reaction proceeds from hematite to magnetite to wüstite to metallic iron.
3Fe2O3 + H2 —> 2Fe3O4 + H2O ∆H973K = -2.17 kJ
When T < 843 K (570 °C)
Fe3O4 + 4H2 à 3Fe + 4H2O ∆H773K = +111.28 kJ
When T > 843 K (570 °C)
Fe3O4 + H2 à 2FeO + H2O ∆H973K = +52.16 kJ
FeO + H2 à Fe + H2O ∆H973K = +15.42 kJ
Suspension reduction
Suspension reduction of iron or suspension iron making or flash iron making refers to the reduction of ultra-fine ground iron ore to metallic iron using hydrogen in a flash reactor at a high temperature of 1500-1800 K. The metallic iron formed can also be melted using external heating and then processed to steel directly inside the flash reactor. This not only eliminates the difficulties associated with coke making, palletization, or sintering in conventional steel making but also reduced the energy needed for further processing of metallic iron in EAF.
Plasma reduction
Hydrogen-based plasma reduction is a modified electric arc furnace that directly produces steel using charges hydrogen plasma in a plasma reactor. During the hydrogen plasma reduction (HPR), a plasma arc region is formed between the hematite and the electrode which reduces the iron ore and simultaneously melts the metallic iron formed thereby yielding molten metal in a single step. This liquid iron can then be processed by adding carbon to produce steel.
Conclusion
Though it is obvious that shifting hydrogen-based reduction techniques can be a sustainable solution to future emission-free steelmaking, there are several challenges associated with implementing them on an industrial scale.
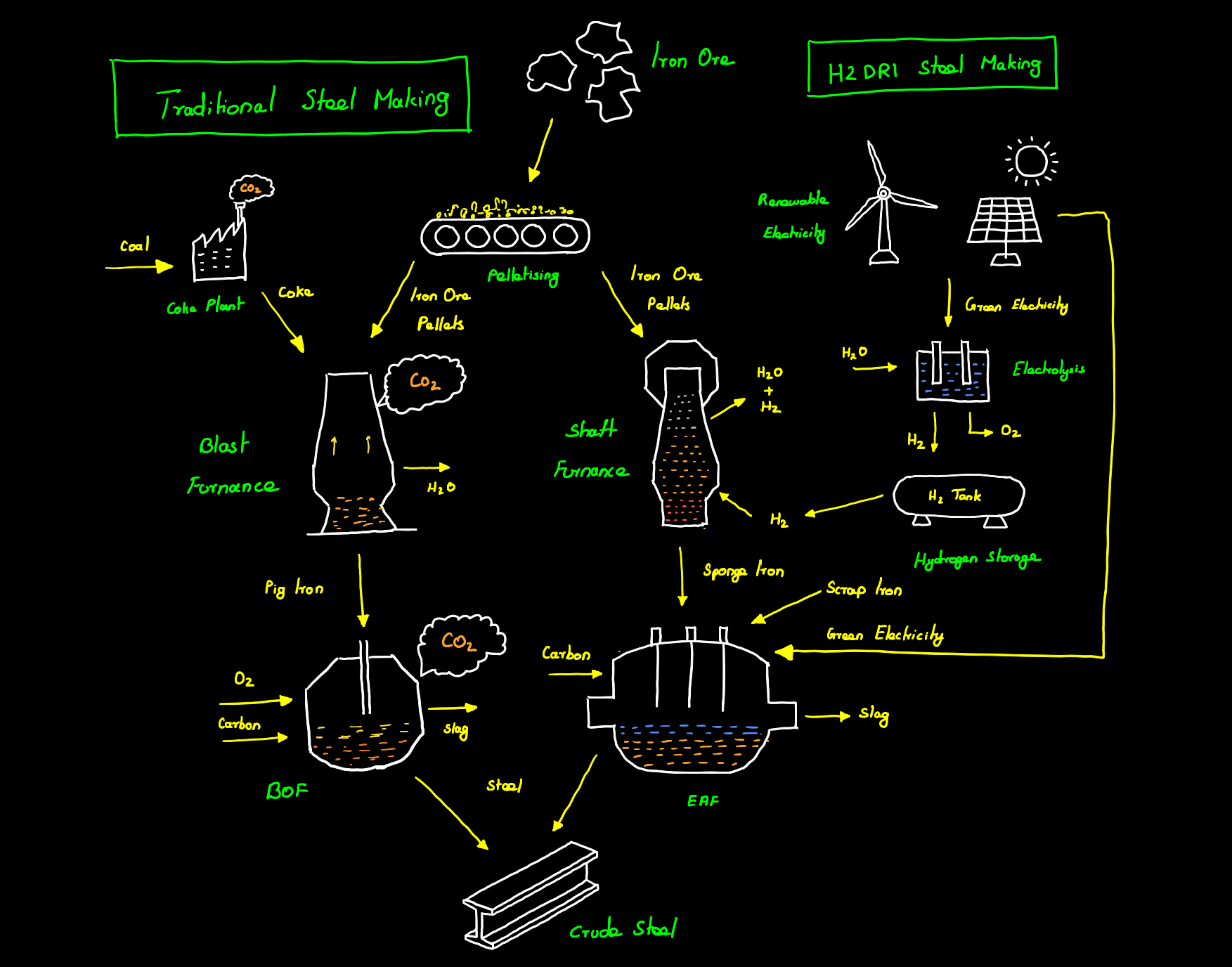
As mentioned above steel industry is the largest consumer of coal and when the shift is made from coal dependence to hydrogen then the steel industry will itself consume a larger portion of the hydrogen being produced. In order to make the entire steelmaking process carbon neutral the steel companies will have to produce their required hydrogen through renewable means and also use renewable energy to power their manufacturing processes. This will increase the operating cost of the entire system which in turn increases the cost of the steel produced. In other words, carbon-neutral steel production will have a higher CAPEX and hence will not be economical unless cheap green hydrogen is available.
— Afrin Hewitt Alban
References
- 1. Europa, B. (2021, May 26). Hydrogen in steel production: what is happening in Europe – part two. Bellona.org. https://bellona.org/news/industrial-pollution/2021-05-hydrogen-in-steel-production-what-is-happening-in-europe-part-two
- Europa, B. (2021a, March 16). Hydrogen in steel production: what is happening in Europe – part one. Bellona.org. https://bellona.org/news/climate-change/2021-03-hydrogen-in-steel-production-what-is-happening-in-europe-part-one
- Europa, B. (2022, May 13). Steel and emissions: How can we break the link? Bellona.org. https://bellona.org/news/ccs/2019-03-is-steel-stealing-our-future
- Dr. Jan-Justus Andrea, Ana Šerdoner, & Keith Whiriskey. (n.d.). An Industry’s Guide to Climate Action. In network.bellona.org. Bellona Europa.https://network.bellona.org/content/uploads/sites/3/2022/05/Industry-Report-final.pdf
- Europe’s steel industry at a crossroads. (2020, April 29). Roland Berger. https://www.rolandberger.com/en/Insights/Publications/Europe's-steel-industry-at-a-crossroads.html
- Hydrogen (H2)-based ironmaking. (n.d.). world steel association. https://worldsteel.org/wp-content/uploads/Fact-sheet-Hydrogen-H2-based-ironmaking.pdf#:~:text=Direct%20reduction%20of%20iron%20is%20the%20chemical%20removal,process%20is%20known%20as%20Direct%20Reduced%20Ironmaking%20%28DRI%29.
- Steel Technology. (2020, August 4). Basic Oxygen Furnace Steelmaking. https://www.steel-technology.com/articles/oxygenfurnace
- What is steel and how is steel made? (n.d.). Retrieved November 4, 2022, from https://www.eurofer.eu/about-steel/learn-about-steel/what-is-steel-and-how-is-steel-made/
- The HYBRIT process. (n.d.). Hybrit. Retrieved November 4, 2022, from https://web.archive.org/web/20201220220736/http://www.hybritdevelopment.com/steel-making-today-and-tomorrow
- Direct reduced iron process. (2021, February 27). Tec-science. https://www.tec-science.com/material-science/steel-making/direct-reduced-iron-dri-process/
- Blast furnace process. (2021, February 27). Tec-science. https://www.tec-science.com/material-science/steel-making/blast-furnace-process/
- Spreitzer, D., & Schenk, J. (2019). Iron Ore Reduction by Hydrogen Using a Laboratory Scale Fluidized Bed Reactor: Kinetic Investigation—Experimental Setup and Method for Determination. Metallurgical and Materials Transactions B, 50(5), 2471–2484. https://doi.org/10.1007/s11663-019-01650-9
- MAJ-BRITT GALDO & ERIKA VESTER. (n.d.). Reduction of iron ore fines in fluidized beds [Degree Project in Technology]. KTH Royal Institute of Technology. http://kth.diva-portal.org/smash/get/diva2:1678309/FULLTEXT01.pdf
- Sohn, H. Y., Fan, D. Q., & Abdelghany, A. (2021). Design of Novel Flash Ironmaking Reactors for Greatly Reduced Energy Consumption and CO2 Emissions. Metals, 11(2), 332. https://doi.org/10.3390/met11020332
- Sohn, H. Y. (2008, March 31). Suspension Hydrogen Reduction of Iron Oxide Concentrates. https://www.osti.gov/biblio/929441
- Souza Filho, I., Ma, Y., Kulse, M., Ponge, D., Gault, B., Springer, H., & Raabe, D. (2021). Sustainable steel through hydrogen plasma reduction of iron ore: Process, kinetics, microstructure, chemistry. Acta Materialia, 213, 116971. https://doi.org/10.1016/j.actamat.2021.116971