Electrochemical Hydrogen Compression

It is well known that hydrogen has the largest energy density in terms of mass and the least energy density in terms of volume, which means burning 1 kg of hydrogen releases more energy compared to 1kg of diesel but burning 1m3 of hydrogen will releases very small energy compared to 1m3 of diesel. This means you need more space to store 1kg of hydrogen than diesel. Hence, this volumetric energy density is an important factor in deciding the storage space of a fuel. In order to increase this volumetric energy density while storing hydrogen in the gaseous form, it is crucial to compress hydrogen. There are several methods of hydrogen compression used today with piston compression being the traditional and the most commercial compression technique. Similarly, there is an equally competitive and more efficient technology that is being developed for hydrogen compression called electrochemical hydrogen compression (EHC) which compresses hydrogen based on the electrochemical principle. This method of hydrogen compression doesn’t require any moving parts, lubrication, or heat management system and hence has increased energy efficiency.
Working principle
Electrochemical hydrogen compression (EHC) consists of an electrolytic cell similar to an electrolyser or fuel cell that has two electrodes separated by an ion exchanging membrane. The hydrogen gas at low pressure is passed to the anode where it undergoes oxidation forming protons and electrons. These protons reach the cathode via a proton exchange membrane (PEM) and the electrons via the external circuit. These electrons and protons undergo a reduction reaction at the cathode and reform hydrogen gas but at higher pressure.
Anode reaction: H2 (low pressure) —> 2H+ + 2e-
Cathode reaction: 2H+ + 2e- —> H2 (high pressure)
Overall reaction: H2 (L.P) —> H2 (H.P)
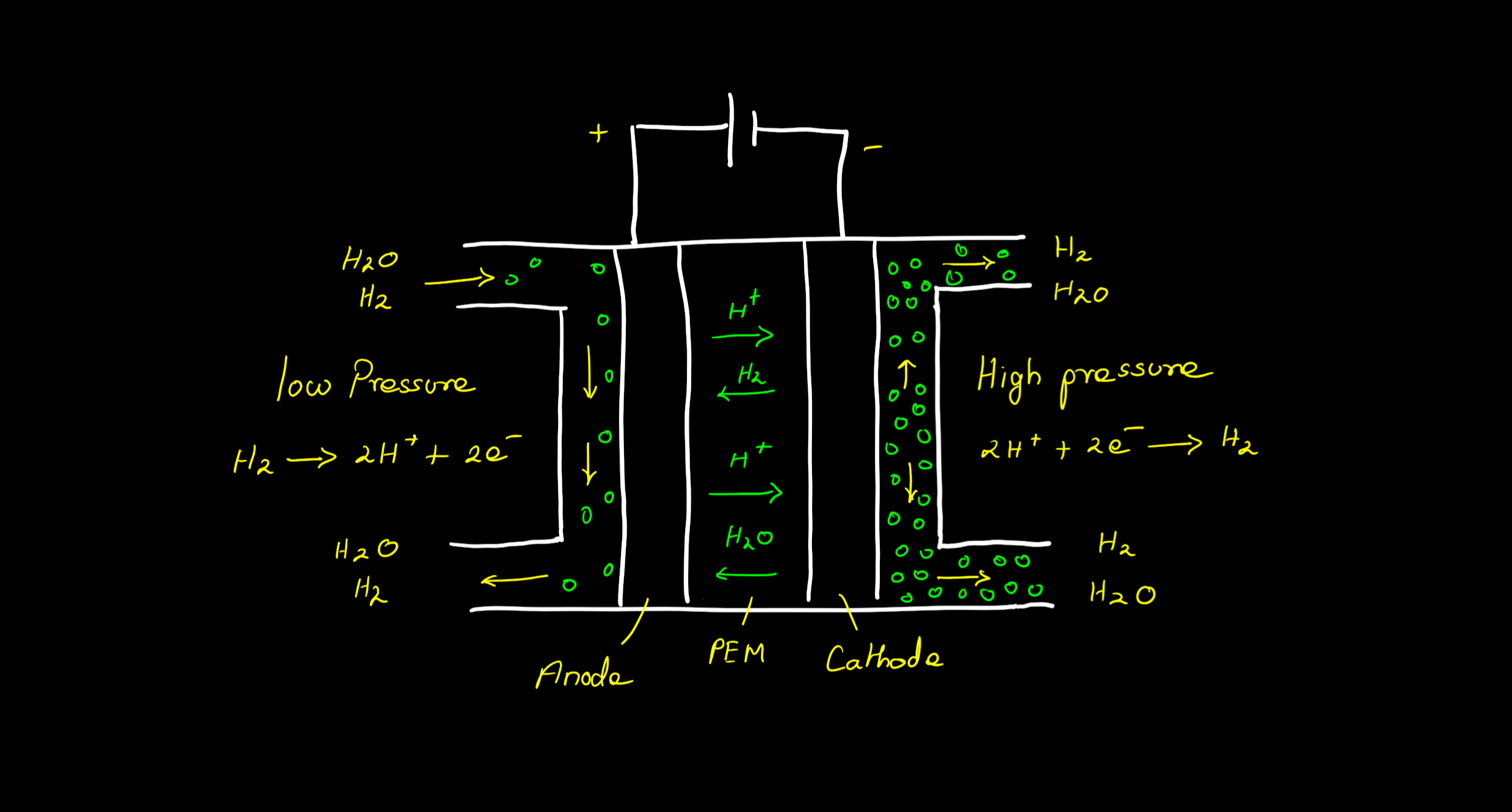
Components of an EHC
The EHC being an electrolytic cell has the same components as an electrolyser or a fuel cell. It is basically a fuel cell that intakes both hydrogen and electric voltage and instead of producing water as output, it produces high-pressure hydrogen gas. The major components include:
1. Proton Exchange Membrane (PEM) that enables the exchange of protons.
· Perfluorosulfonic acid (PFSA) based membrane like Nafion 117.
· Hydrocarbon-based membranes like Polyether Ether Ketone (PEEK)
membranes or Sulfonated Polyether Ether Ketone (SPEEK).
· Protonic Ceramic membranes for high-temperature EHC like LBYb-91.
· Ion-Solvating Polymers like Phosphoric acid doped Polybenzimidazole (PBI).
2. Electrocatalysts that perform the oxidation and reduction reaction.
3. Flow field that enables mass distribution and transportation in the porous electrodes with minimal pressure drops.
4. Gas diffusion layer which is a porous structure that enables the diffusion of reactants, water, heat, and electrons.
Types of EHC
EHC is classified based on the temperature of operation as low-temperature and high-temperature electrochemical hydrogen compressors. Low-temperature compressors operate at less than 100 °C and are mostly based on PEMs. High-temperature electrolysers operate at temperatures greater than 100 °C and are mostly based on solid oxide membranes.
State of the art
Though this technology is today on a lab scale there are several companies working on it:
1) The HyET Group, Netherlands: The HyET HCS100 EHC has a compression capability of up to 2000 kg per day and could compress hydrogen on a single stage of more than 900 bar.
2) Kaji technology and Toray from Japan in the year 2021 have together demonstrated electrochemical hydrogen compression with PEM which they call it the electrochemical hydrogen pump system and is capable of pumping hydrogen at 196 bar.
3) Skyre Inc, United States has developed an electrochemical hydrogen compressor called H2RENEW which was originally developed for NASA as part of critical life support systems for astronauts.
References:
1) Han, N., Yan, J., Feng, Q., Wang, Y., Zhao, Z., Fan, J., Zeng, L., Li, H., & Wang, H. (2020, August 7). Electrochemical Compression Technologies for High-Pressure Hydrogen: Current Status, Challenges, and Perspective. Electrochemical Energy Reviews, 3(4), 690–729. https://doi.org/10.1007/s41918-020-00077-0
2) L. L. (n.d.). Electrochemical Hydrogen Compressor [Slide show]. FuelCell Energy, Inc. https://www.hydrogen.energy.gov/pdfs/review12/pd048_lipp_2012_o.pdf
3) Nel Hydrogen. (2020, April 2). What is Electrochemical Compression? AZoM.Com. Retrieved September 8, 2022, from https://www.azom.com/article.aspx?ArticleID=16967
4) Grigoriev, S & Dzhus, Kirill & Millet, P & Kalinnikov, A. & Porembskiy, V. & Fateev, Vladimir & Blach, R & Sq, Kurchatov. (2008). Characterization of PEM electrochemical hydrogen compressors. Fundamentals and Developments of Fuel Cells Conference FDFC, Nancy, France.
5) Kaji Technology and Toray Complete the Development and Demonstration of Electrochemical Hydrogen Pump System under the NEDO Project “Development of technologies for Hydrogen Refueling Stations” toward the Realization for a Carbon Neutral Society | Latest News | Toray Industries, Inc. | TORAY. (n.d.). TORAY INDUSTRIES, INC. Retrieved September 8, 2022, from https://www.toray.com/global/news/details/20210331000598.html
6) SKYRE Announces The World's First Commercially Available High-Pressure Electrochemical Hydrogen. (2019, April 20). FuelCellsWorks. Retrieved September 8, 2022, from https://fuelcellsworks.com/news/skyre-announces-the-worlds-first-commercially-available-high-pressure-electrochemical-hydrogen-separation-and-compression-system/
7) Suermann, M., Kiupel, T., Schmidt, T. J., & Büchi, F. N. (2017). Electrochemical Hydrogen Compression: Efficient Pressurization Concept Derived from an Energetic Evaluation. Journal of the Electrochemical Society, 164(12), F1187–F1195. https://doi.org/10.1149/2.1361712jes
8) Bampaou, Michael & Panopoulos, Kyriakos & Papadopoulos, Athanasios & Seferlis, Panagiotis & Voutetakis, Spyros. (2018). An Electrochemical Hydrogen Compression Model. Chemical Engineering Transactions. 70. 1213-1218. 10.3303/CET1870203.