Ammonia as a hydrogen carrier

When the entire world agreed together in fighting climate change with the signing of the Paris agreement, the International Maritime Organisation (IMO) also made strict regulations on the reduction of PM, SOx, and NOx emissions. The IMO has hence decided to reduce the emission of greenhouse gases by 50% compared to that in the year 2008. The international carbon emission from the shipping industry in the year 2008 accounted for about 916 million tonnes and in this current emission rate, the carbon emission will be more than 3000 million tonnes by the year 2050. Hence it is really necessary to reduce the emission of carbon dioxide. This has led the manufacturers and companies working in the marine transport industry to explore several other green fuels for future marine transportation. In this regard hydrogen as a fuel seems more promising than other available green fuels especially due to the growing interest and development in the hydrogen economy. The marine transport ecosystem itself is large and more demanding than other means of transport, as it’s been one of the major mediums of goods transport for centuries. The technological difficulties in handling hydrogen, especially the storage of hydrogen in large quantities have enabled the exploration of alternate ways of storing and efficiently using this fuel. In this regard, Ammonia seems a more promising medium of hydrogen storage and transport. This article gives an idea about the feasibility of using ammonia as a hydrogen carrier for use in ships.
What makes ammonia a hydrogen carrier?
1. Ammonia can be stored as a liquid at -34 °C and 10 bar. Hence, less energy is needed for liquefaction.
2. The volumetric density of liquid ammonia is 12.7 MJ/liter which is greater than liquid hydrogen (8.5 MJ/liter) and compressed gas at a pressure of 690 bar (4.7 MJ/liter).
3. Ammonia has the largest storage density of other storage mediums. One cubic meter of ammonia could store 123 kg of hydrogen.
4. When an electrolyzer and ammonia synthesis plant is provided with renewable electricity, ammonia can be produced without greenhouse gas emissions.
5. Ammonia can be easily cracked to release hydrogen which can be then physically or chemically combusted to produce energy.
6. As ammonia is already being used in the making of fertilizers there is a well-established supply chain for ammonia production and transport. There are about 120 ports internationally, capable of handling ammonia export and import.
Hydrogen to ammonia.
Traditionally ammonia is synthesized from natural gas or coal based on the Haber-Bosch process. The hydrogen produced by reforming natural gas or coal gasification is made to react with nitrogen from air under high temperature (300-400 °C) and high pressure (~200 bar) under the presence of iron or ruthenium-based catalyst.
N2 + 3H2 --> 2NH3 (∆H=-92.4 kJ/mol)
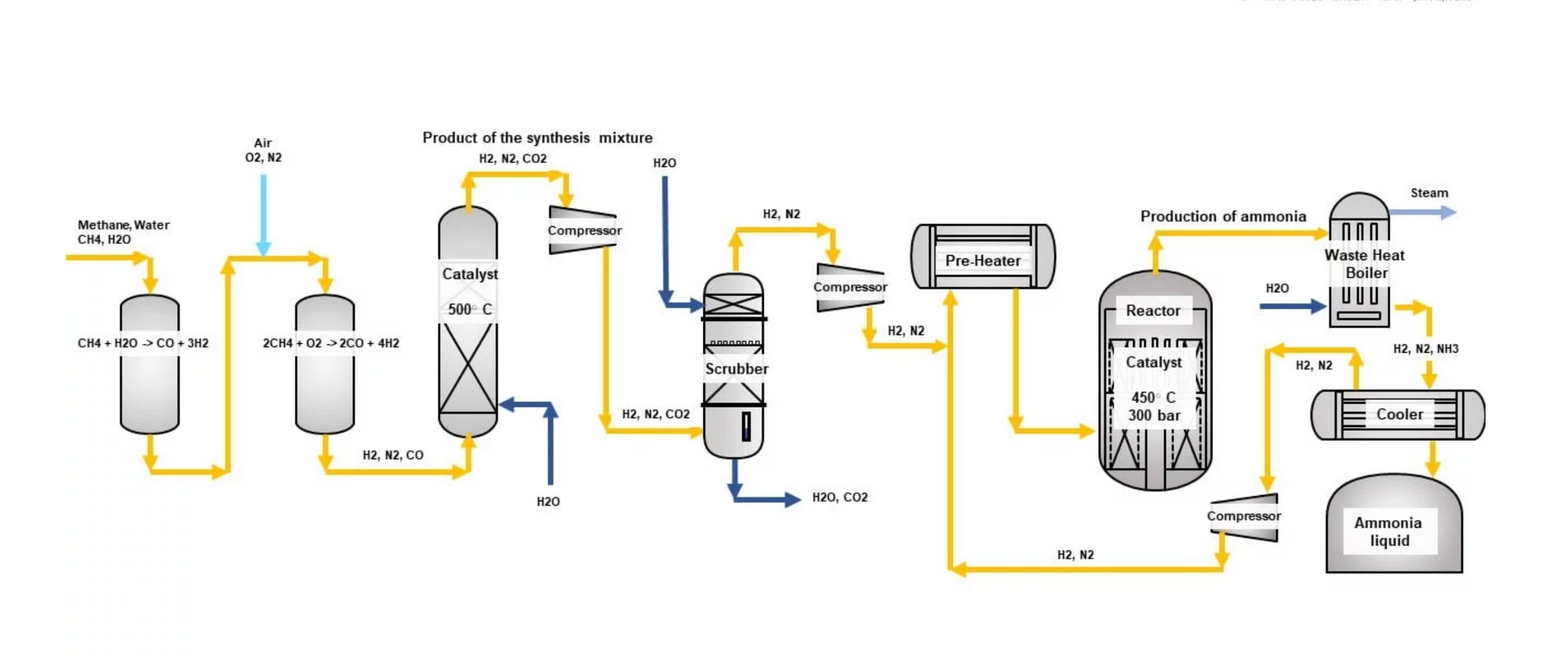
However, this method of ammonia production leads to the emission of carbon dioxide and hence ammonia has to be produced through the electrical synthesis of green hydrogen and nitrogen separated from the air using an electrolytic cell. This is called electrochemical ammonia synthesis.
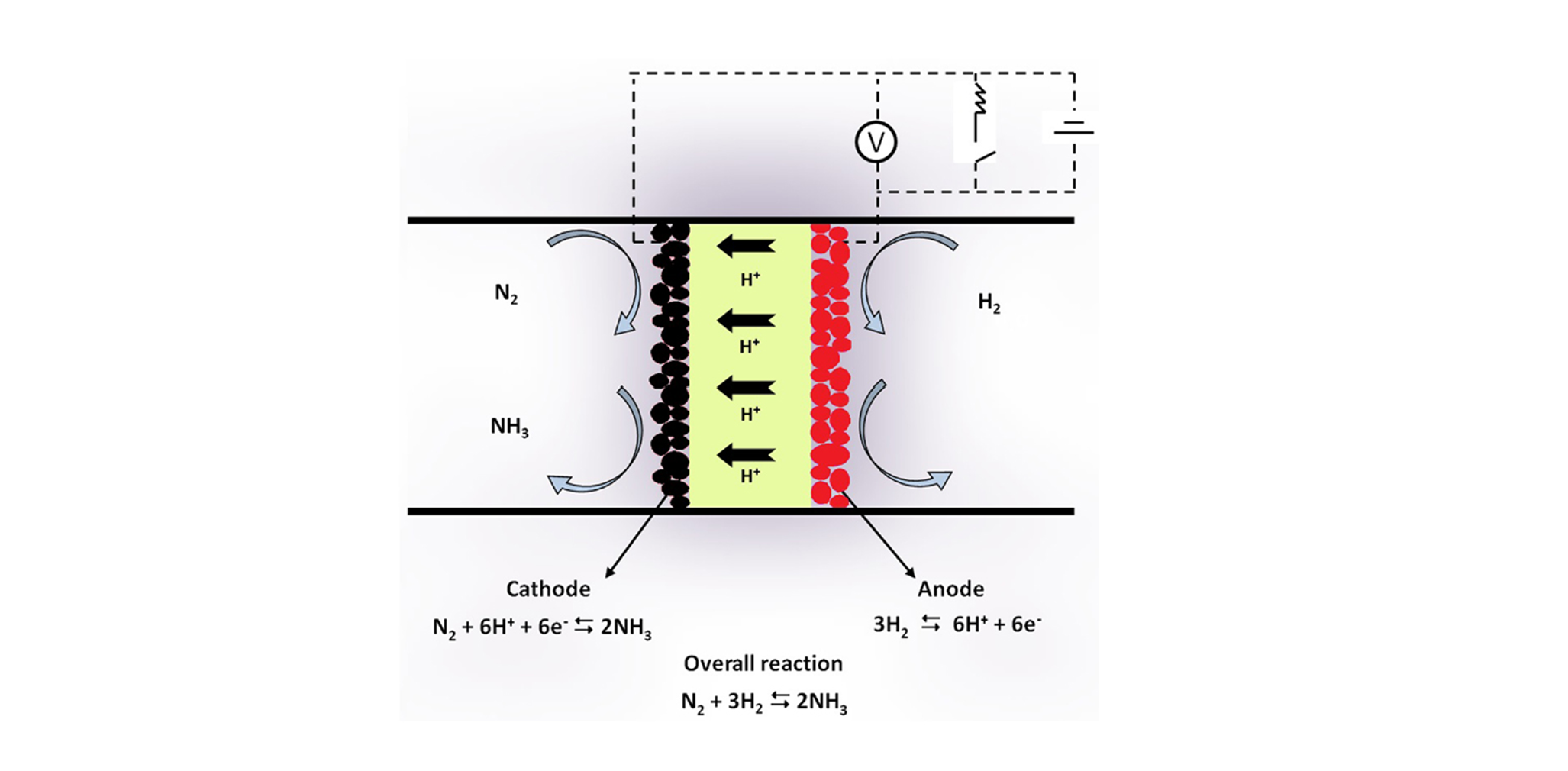
Ammonia to hydrogen
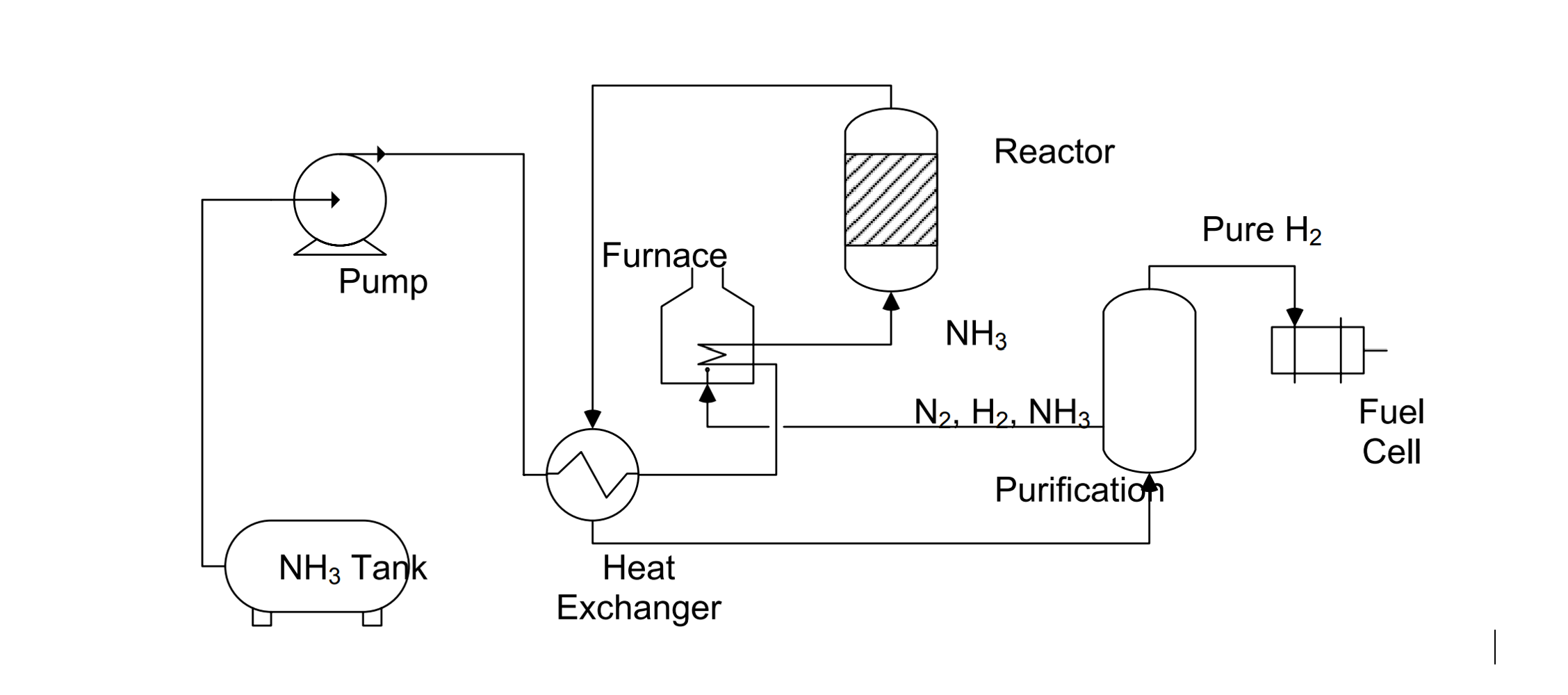
Ammonia itself can burn in the air due to the higher hydrogen content. However direct combustion of ammonia will lead to higher emissions of NOx which is a harmful greenhouse gas. Hence the usage of post-capturing techniques like selective catalytic reduction of NOx is necessary. Therefore, the most efficient and eco-friendly method of using ammonia is to convert it to hydrogen using the process called ammonia cracking where ammonia at high temperature (650-700 °C) is broken down into hydrogen and nitrogen.
2NH3 --> N2 + 3H2 (∆H=+46 kJ/mol)
Why is this path being considered?
Hydrogen is regarded as the most promising emission-free fuel of the future, but its small volumetric energy density compared to conventional fossil fuels makes it challenging to be used in the marine industry. In order to increase the volumetric density of hydrogen, it must either be compressed or liquefied which are both expensive and energy consuming. Ships are the cheapest mode of transporting goods across continents but the combustion engines that propel these ships burn large volumes of fuel. If these ship engines were to burn hydrogen in the future, a large space on board will be used for hydrogen storage alone. To tackle this problem hydrogen carriers are being used. These elements carry large volumes of hydrogen and require less storage space. In this regard, Ammonia seems to be a more promising energy carrier. Green hydrogen can be stored in the form of ammonia and stored at the ports. This ammonia can then be filled into the fuel tanks of the ships. This ammonia can then be cracked on board to release the stored hydrogen, which can then be used to propel the ship.
Bibliography:
1) Garagounis, I., Kyriakou, V., Skodra, A., Vasileiou, E., & Stoukides, M. (2014). Electrochemical Synthesis of Ammonia in Solid Electrolyte Cells. Frontiers in Energy Research, 2. https://doi.org/10.3389/fenrg.2014.00001.
2) Short History of Ammonia Process – Past, Present and Future – AmmoniaKnowHow. (2022, June 25). Retrieved September 5, 2022, from https://ammoniaknowhow.com/short-history-of-ammonia-process-past-present-and-future/
3) Thomas, G., & Parks, George . (n.d.). Potential Roles of Ammonia in a Hydrogen Economy . U.S. Department of Energy. https://www.energy.gov/sites/prod/files/2015/01/f19/fcto_nh3_h2_storage_white_paper_2006.pdf
4) Report on Ammonia-Fueled Ships. (n.d.-a). Korean Register. https://safety4sea.com/wp-content/uploads/2021/02/Korean-Register-Report-on-Ammonia-Fueled-Ships-2021_02.pdf
5) Chambers, S. (2020, February 18). Ammonia’s advantages detailed in new report. Splash247. Retrieved September 5, 2022, from https://splash247.com/ammonias-advantages-detailed-in-new-report/
6) Amogy. (2022). Ammonia as a Fuel to Decarbonize Transportation. Guidehouse Inc.